Malaria.com is delighted to present this latest e-issue in partnership with WWARN (the WorldWide Antimalarial Resistance Network). WWARN focuses on gathering data and collating resources related to malaria medication, and specifically factors affecting its efficacy. READ MORE>>
Blog Posts
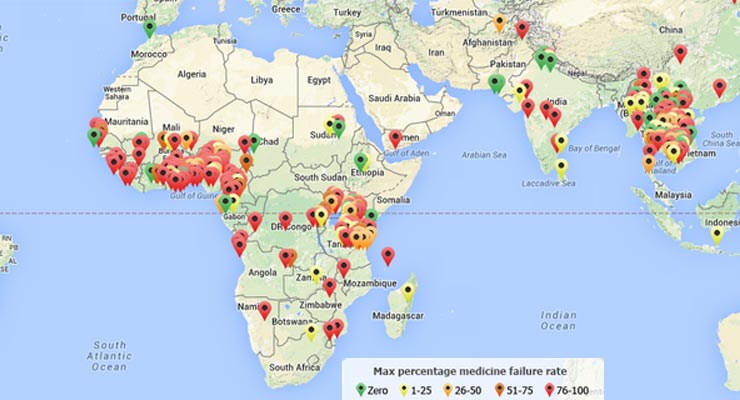
Antimicrobial Resistance: The Silent Threat in Malaria Eradication
By Bernard Owusu Agyware, Arielle Dolegui and Jesus Aquilar Malaria is a serious and sometimes deadly disease, affecting millions and claiming more than 600,000 lives worldwide each year in 2022 and 2021.1 Low and Middle-Income Countries (LMICs), particularly in Sub-Saharan Africa, disproportionately bear the greatest burden of malaria. The fight to eradicate this worrisome disease […]

Shouldering the burden: determinants of malaria in Nigeria and implications for future interventions
By Charlotte Follari, Norma Quintanilla, and Emily Shambaugh Malaria is one of the world’s most widespread and deadly infectious diseases, with nearly half of the world’s population at risk of exposure. One of the major barriers to effective malaria control, and the reason why it remains such an entrenched public health problem, is that risk […]
NEWS
Challenges for Malaria Elimination: Cycles of Poverty
By Corey Rathe, Sabrina Barrett, Hannah Hidle, Emma Weimer Malaria is a serious and life-threatening disease – it is also preventable and treatable. So, why does malaria continue to devastate the lives of hundreds of millions each year? Poverty cycles hold much of this responsibility. Disease and suffering are amplified through overwhelming and cyclical traps. […]
Getting to Know Plasmodium knowlesi: The New Parasite on the Block
By Matt Boyce, Gretchen Mohr, and Eva Rest Plasmodium knowlesi, one species of the multiple protozoan parasites that cause malaria, has joined the lineup of human malaria parasites. P. knowlesi was originally known to cause malaria in long-tailed and pig-tailed macaques typically found in Southeast Asia (Figure 1). Only within the last two decades have […]
New Research May Lead to Rapid Screening Test for Subclinical Infections
While the global health community has made great strides toward eradicating malaria through prevention and treatment strategies, rapid and inexpensive methods to diagnose submicroscopic malaria in individuals who have no clinical symptoms and undetectable levels of disease-causing parasites in their blood remain an unmet need. New research could pave the way for a rapid screening test capable of diagnosing submicroscopic infections
WHO: Urgent Call for End to Malaria Resurgence
The World Health Organization is joining a worldwide call to stop a resurgence of malaria that threatens much of the progress made over the past decade. To mark World Malaria Day, WHO is pushing for urgent action – and money – to get the global fight against this ancient scourge back on track.
Four Year Old Dies of Malaria in Italy
A four-year old girl has died of a severe form of malaria contracted in Italy, where the disease is supposed to have been eradicated years ago nearly half a century ago. Italy’s health ministry on Tuesday said it was sending experts to investigate the death Sofia Zago, who died in hospital in the northern city of Brescia overnight between Sunday and Monday.
Research
KSHV Oral Shedding and Plasma Viremia Result in Significant Changes in the Extracellular Tumorigenic miRNA Expression Profile in Individuals Infected with the Malaria Parasite
In the current study, the host and viral extracellular mature miRNA expression profiles were analyzed in blood of KS-negative individuals in Uganda, comparing those with or without KSHV detectable from the oropharynx.
Tools and Strategies for Malaria Control and Elimination: What Do We Need to Achieve a Grand Convergence in Malaria?
Progress made in malaria control during the past decade has prompted increasing global dialogue on malaria elimination and eradication. The product development pipeline for malaria has never been stronger, with promising new tools to detect, treat, and prevent malaria, including innovative diagnostics, medicines, vaccines, vector control products, and improved mechanisms for surveillance and response.
Does High-Dose Antimicrobial Chemotherapy Prevent the Evolution of Resistance?
High-dose chemotherapy has long been advocated as a means of controlling drug resistance in infectious diseases but recent empirical studies have begun to challenge this view. Researchers have developed a general framework for modeling and understanding resistance emergence based on principles from evolutionary biology.
Targeting the Cell Stress Response of Plasmodium Falciparum to Overcome Artemisinin Resistance
Successful control of falciparum malaria depends greatly on treatment with artemisinin combination therapies. Thus, reports that resistance to artemisinins (ARTs) has emerged, and that the prevalence of this resistance is increasing, are alarming. The researchers’ model predicts that extending current three-day ART treatment courses to four days, or splitting the doses, will efficiently clear resistant parasite infections.
Challenges in Modelling Infectious DIsease Dynamics
Malaria, HIV, and tuberculosis (TB) collectively account for several million deaths each year, with all three ranking among the top ten killers in low-income countries. Despite being caused by very different organisms, malaria, HIV, and TB present a suite of challenges for mathematical modellers that are particularly pronounced in these infections, but represent general problems in infectious disease modelling, and highlight many of the challenges described throughout this issue. Here, we describe some of the unifying challenges that arise in modelling malaria, HIV, and TB, including variation in dynamics within the host, diversity in the pathogen, and heterogeneity in human contact networks and behaviour. Through the lens of these three pathogens, we provide specific examples of the other challenges in this issue and discuss their implications for informing public health efforts.