Finding out what medicines contain and investigating their packaging are vital steps in the fight against poor quality medicines, both substandard and falsified. Falsified medicines usually masquerade as real medicines by copying the appearance and packaging of the real product, but may not contain any of the same active ingredients, or in the correct amounts. Substandard medicines result from errors/negligence within factories and usually result in medicines with too little or too much active ingredient. We briefly describe here selected technologies and devices used (or under development) in the different steps of medicine quality analysis. Interesting reviews are available elsewhere for those looking for more detailed information.1–3
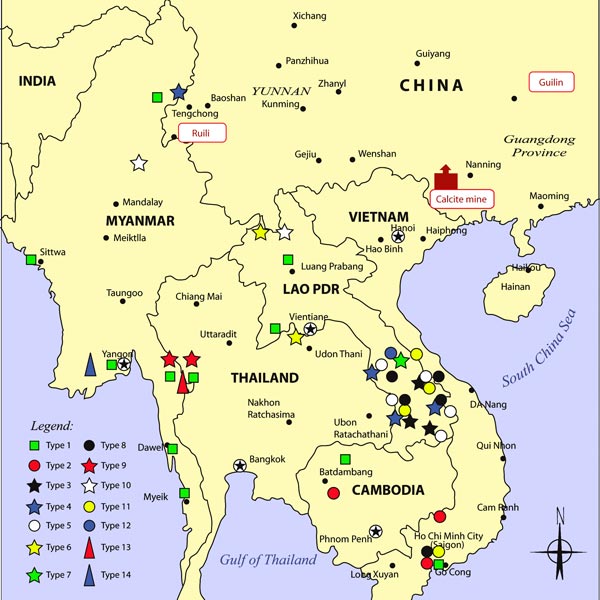
Map of the Distribution of Fake Artesunate, Collected by Wellcome Trust-University of Oxford SE Asian Tropical Medicine Research Programme and Collaborators, in Relation to Packaging Type. Map drawn by Mr. Chongkham Phonekeo.
Source: PLOS Medicine – DOI: doi:10.1371/journal.pmed.0050032.g001 (Creative Commons license).
Packaging analysis
Basic visual analysis of the packaging is the most inexpensive way to rapidly identify the more crudely presented falsified medicines. Comparing packaging with that of authentic examples can help identify imitation packaging, but it is usually very difficult to obtain the authentic medicines from legitimate manufacturers for comparison.
Observing the uniformity of the physical characteristics of the tablets/capsules in the same sample (size, shape, odour, and taste) can raise suspicions about a product’s authenticity, and is a technique that can be used at every point of the supply chain with minimal training and cost. A useful checklist for visual inspection of medicines has been developed by the International Council of Nurses in partnership with the United States Pharmacopoeia.4
In addition to its primary function of detecting correct active pharmaceutical ingredients via colorimetric or chromatographic methods, the German Pharma Health Fund (GPHF) MiniLab (see below) provides a physical inspection scheme of dosage forms and associated packaging material.5
With increased sophistication in falsification, more sophisticated techniques are required to allow the detection of anomalies in packaging or products that are not apparent to the naked eye. The handheld Counterfeit Detection Device, version 3 (CD3), developed by the US Food and Drug Administration uses a variety of different wavelengths of light (from the ultraviolet to the infrared) to highlight differences between falsified tablets/capsules and packaging and authentic samples.6 Falsified artesunate was an enormous public health problem in Southeast Asia until recently. The CD3 device has shown very good accuracy in detecting falsified artesunate packaging and tablets, but its ability to identify substandard medicines still needs to be evaluated.7
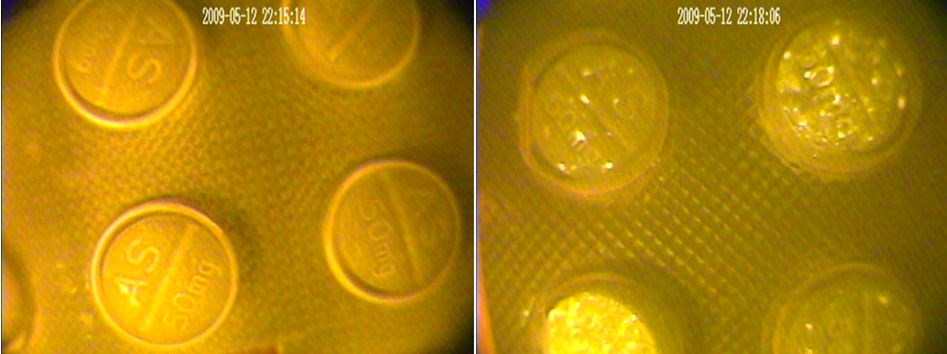
The genuine tablets on the left show the homogeneous, or standardized, quality of the excipients whereas the falsified tablets on the right show their heterogeneous, variable quality. Source: FDA (Forensic Chemistry Center) – Centers for Disease Control & Prevention – National Institutes of Health.
Active ingredient analysis
Techniques ranging from the simple to the very sophisticated have been developed to identify and quantify various active pharmaceutical ingredients (APIs).
Colorimetric tests, which produce distinct colour changes when a particular active ingredient in a medicine reacts with a specific reagent, provide rapid qualitative information on the presence of the specified active ingredient; semi-quantitative or quantitative information can also be obtained in some cases. Developed for resource-limited settings, the GPHF-Minilab, a portable laboratory consisting of two suitcases, provides simplified colour reaction tests for 80 essential medicines, mainly anti-infectives.
Conventional techniques used in a laboratory to assess the quality of a medicine are high performance liquid chromatography (HPLC) and mass spectrometry. The latter also allows the investigators to look for unexpected wrong ingredients in medicines. However, these technologies are costly, require sample preparation, skilled technicians, quality control and a steady electricity supply, and are thus not adapted for fieldwork.
Portable technologies may be useful for testing medicine quality at different points in the pharmaceutical supply chain. The Minilab colorimetric and TLC tests, although requiring minimal training, require sample preparation and only reliably detect falsified and grossly substandard medicines. It is therefore recommended to use these tests in conjunction with other more sophisticated techniques when poor quality medicines are suspected.8,9 Recently developed colour reactions on paper test cards also allow sensitive, inexpensive (0.50 $ per test card), rapid (10 minutes) and easy-to-use screening of the composition of a wide range of anti-infectives outside the laboratory.10,11
New portable, battery-powered devices, based on Raman and near infrared spectroscopic technologies, do not require sample preparation, give relatively fast results, and require no extra supplies and minimal training. Unfortunately, the price of the devices currently available is prohibitive for medicine inspectors in low- or middle-income countries, and there have been no comparisons of their diagnostic accuracy and cost-effectiveness for diverse medicines. PharmaCheck is a portable microfluidics device under development that allows specific and quantitative measurements of active ingredients and medicine release over time.12
Assessment of the bioavailability
The degree to which an oral medicine becomes absorbed into the blood is a measure known as ‘bioavailability’. When incorrect excipient formulations are used, this may result in reduced dissolution of the medicine in the gastrointestinal tract. In turn, this results in a reduction of the concentration of the active ingredients in the blood stream, which consequently decreases the efficacy of the medicine. For example, with poor bioavailability of an antimalarial, malaria parasites will ‘see’ concentrations of the active drug that are too low.
Reference tests for dissolution assessment, that measure how fast the active ingredient is solubilized in fluid, are costly, time-consuming, and require trained staff. The GPHF-Minilab provides a simple tablet and capsule disintegration test. As far as we know, the only field-adapted device is the PharmaCheck device currently under development.
Forensics
A wide range of forensic investigations can be pursued in order to give clues as to the origin of falsified medicines.3 However, the process of forensic analysis usually requires a network of collaborating laboratories, as the techniques are costly and/or time-consuming, and include: examination of paper type and printing techniques, microscopic investigation of debris and pollen in tablets; isotope ratio mass spectrometry to potentially identify excipients and pinpoint their geographical origin; and X-ray fluorescence to investigate elemental composition.
Conclusion
In conclusion, new techniques to identify poor quality medicines are available and it is a fertile research field. However, many are currently too costly for low-resource countries, not field-adapted (although new handheld detection technologies are promising) or have not been tested extensively outside a research laboratory setting, cannot detect substandard medicines containing low doses of active pharmaceutical ingredients, and require trained users.
There is unlikely to be a single tool to test all aspects of medicine quality throughout the supply chain. Moreover, there is no clear global vision regarding which tests are reliable for which medicines at which point. There is no accurate and reliable device to distinguish degraded medicines from those which left the factory as substandard, containing a low amount of API resulting from negligence or errors made during the manufacturing process. This means that not only detecting, but knowing how best to address, the issue of poor quality medicines is a significant challenge, and one which has a significant impact on health and wellbeing worldwide. This topic is addressed in more detail in the third article of this e-issue.
Other articles in this series:
- Five Things You Might Not Know About Medicine Quality
- How Can We Detect Poor Quality Medicines?
- Challenges in Tackling the Issue of Poor Quality Medicines
- Hot Spots: Recent Evidence on the Quality of Currently Recommended Antimalarials
References
- Kovacs, S. et al. Technologies for detecting falsified and substandard drugs in low and middle-income countries. PLoS One 9, e90601 (2014).
- Martino, R., Malet-Martino, M., Gilard, V. & Balayssac, S. Counterfeit drugs: analytical techniques for their identification. Anal. Bioanal. Chem. 398, 77–92 (2010).
- Fernandez, F. M. et al. Poor quality drugs: grand challenges in high throughput detection, countrywide sampling, and forensics in developing countries. Analyst 136, 3073–82 (2011).
- International Council of Nurses, U. S. P. Tool for Visual Inspection of Medicines. at <http://www.usp.org/sites/default/files/usp_pdf/EN/dqi/visualInspectionTool.pdf>
- The GPHF-MinilabTM – Protection Against Counterfeit Medicines. at <http://www.gphf.org/en/minilab/index.htm>
- U.S. Food and Drug Administration. FDA Facts: FDA’s Counterfeit Detection Device CD-3. at <http://www.fda.gov/downloads/NewsEvents/Newsroom/FactSheets/UCM349286.pdf>
- Ranieri, N. et al. Evaluation of a new handheld instrument for the detection of counterfeit artesunate by visual fluorescence comparison. Am. J. Trop. Med. Hyg. 91, 920–4 (2014).
- Risha, P. G. et al. The use of Minilabs to improve the testing capacity of regulatory authorities in resource limited settings: Tanzanian experience. Health Policy 87, 217–22 (2008).
- Bate, R. & Hess, K. Anti-malarial drug quality in Lagos and Accra – a comparison of various quality assessments. Malar. J. 9, 157 (2010).
- Weaver, A. A. & Lieberman, M. Paper test cards for presumptive testing of very low quality antimalarial medications. Am. J. Trop. Med. Hyg. 92, 17–23 (2015).
- Weaver, A. A. et al. Paper analytical devices for fast field screening of beta lactam antibiotics and antituberculosis pharmaceuticals. Anal. Chem. 85, 6453–60 (2013).
- PharmaChk: Substandard and Counterfeit Medicines Rapid Detection and Screening Platform | Saving Lives at Birth. at <https://savinglivesatbirth.net/summaries/327>
Leave a Reply
You must be logged in to post a comment.