By Bernard Owusu Agyware, Arielle Dolegui and Jesus Aquilar
Malaria is a serious and sometimes deadly disease, affecting millions and claiming more than 600,000 lives worldwide each year in 2022 and 2021.1 Low and Middle-Income Countries (LMICs), particularly in Sub-Saharan Africa, disproportionately bear the greatest burden of malaria. The fight to eradicate this worrisome disease has made significant progress since 1955 when the first global malaria eradication program (GMEP) was launched.2 Spanning over half a century, many techniques have been developed to tackle malaria, including treated bed nets, indoor residual spraying, and improved case management (notably diagnostics and treatment). In addition, mass malaria education has contributed significantly to malaria reduction. The recent approval and use of malaria vaccines hold great promise in this global endeavor. Yet, much more needs to be done since the vision of the World Health Organisation (WHO) and the global malaria community is a world free of malaria.3 Despite the success so far, a substantial threat to malaria eradication is on the horizon. Antimicrobial or, more specifically, Antimalarial Resistance (AMR), is threatening to undo decades of hard work in malaria control.
Before delving into AMR and its implications for malaria eradication, it is important to understand the basics of the disease. Malaria is a mosquito-borne disease caused by a parasite called Plasmodium. It is transmitted to humans through the bite of infected mosquitoes. The parasite goes through different stages of development, first in the human liver, and subsequently infects red cells. Infected red cells burst and release more parasites to infect other red cells in a cyclical pattern. The bursting of the cells and the activation of the human immune mechanisms lead to symptoms such as fever, chills, headache, muscle aches, and tiredness. If the disease is not treated promptly, it can lead to severe complications and ultimately death.
Next-generation antimalarial drugs, such as artemisinin-based combination therapies (ACTs), have been vital in treating malaria.4 Currently, they are the most effective drugs against malaria parasites. They work by killing the malaria parasites in the bloodstream. Before ACTs, quinine-based drugs (chloroquine) were the preferred malaria chemotherapy until resistance emerged in Southeast Asia and South America in the late 1950s and early 1960s.5 Chloroquine-resistant parasites spread to all corners of the globe and resulted in increased malaria diseases and deaths in the ensuing years. This development seriously undermined global malaria control efforts until artemisinin was discovered in the 1970s. Artemisinin proved effective in malaria treatment and has since 2005 been recognized by the World Health Organization (WHO) as the preferred malaria drug therapy of choice. However, the emergence of artemisinin resistance in Southeast Asia and other parts of the world implies that a similar fate as chloroquine resistance awaits humanity if proactive measures are not pursued.
AMR occurs when microorganisms, like the malaria parasite, change and evolve to become resistant to the drugs designed to get rid of them. The mechanisms behind AMR are complex, but they can result from factors such as improper drug use, overuse of dosing, and low-quality or counterfeit medications.
AMR emergence has multiple implications for malaria eradication. The first of these concerns is the efficacy of malaria treatments. As resistance to key antimalarials spreads, the very tools we rely on to fight malaria become less effective. This diminished antimalarial efficacy poses a threat to our ability to control and treat malaria. When malaria parasites develop resistance to antimalarials, treatment failure becomes more likely, and can lead to repetitive episodes of disease and subsequent re-treatment. As a result, the infected individuals face prolonged illness and a higher risk of death.
Furthermore, the economic impact of antimalarial resistance is another significant concern. With extended treatment durations and more severe cases resulting from AMR, the burden on healthcare systems and economies is likely to increase. Longer and more intensive treatment regimens not only demand more resources but also have broader economic consequences, affecting productivity and overall human potential.
Beyond the immediate health and economic concerns, AMR in malaria has implications for global health security. Widespread international travel can facilitate the global spread of malaria-resistant strains. This will be especially concerning for populations living in regions non-endemic for malaria. To protect the health of its citizens, governments may impose travel restrictions and point-of-entry health screening measures, particularly for people traveling from malaria-endemic regions. This will impact trade, business, and tourism.
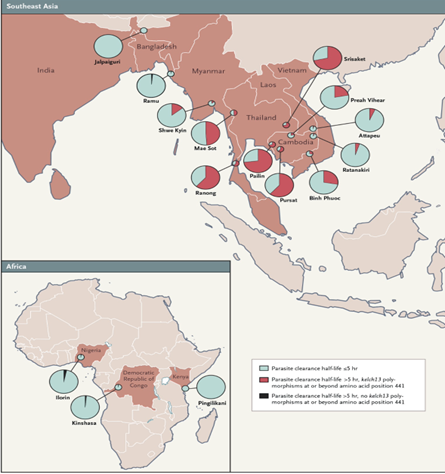
Figure 1. Geographical Distribution and Proportion of Patients with Artemisinin Resistance Based on a Research Study.6
As shown in Figure 1, artemisinin resistance is now present in SEA and reported in Part of Africa Artemisinin resistance is now present in all six WHO regions, but it is most prevalent in Southeast Asia and the Greater Mekong sub-region. The spread of artemisinin resistance is a major threat to global malaria control and elimination efforts. To combat AMR in malaria, a multifaceted approach is essential. Primarily, we focus on a strategy we define as CD3. This strategy requires that the correct drugs are prescribed with the right dose and duration. Proper drug use is a fundamental component of slowing the development of resistance. This strategy must be pursued in tandem with widespread education on the dangers and consequences of self-medication.
Investment in research and development is crucial for providing alternative treatment options when resistance occurs. Developing new antimalarials that remain effective against resistant strains is a key strategy in the fight against AMR. Regular surveillance and monitoring of resistance patterns are vital components of a proactive response. These measures help guide treatment strategies and allow for early detection of emerging resistant strains.
Lastly, vector control remains extremely important. Preventing the transmission of malaria through mosquito control measures remains crucial for malaria eradication efforts. By reducing the transmission of the disease, we can also reduce the selective pressure that drives the emergence of resistance.
In conclusion, the implications of AMR in malaria are a threat and demand a prompt response. The ongoing fight against this ancient and deadly disease needs vigilance, innovation, and a commitment to preserving the effectiveness of our antimalarial tools. With a comprehensive strategy that encompasses proper drug use, research and development, surveillance, and vector control, we can achieve a malaria-free world.
References
- World Malaria Report 2022. Geneva: World Health Organization; 2022. License: CC BY-NC-SA 3.0 IGO.
- WHO. Geneva: World Health Organization; 1973. Malaria. Handbook of resolutions and decisions of the World Health Assembly and the Executive Board. Volume I, 1948–1972, 1st to 25th WHA and 1st to 50th EB. pp. 66–81.
- Global technical strategy for malaria 2016-2030. Geneva: World Health Organization; 2015
- Eastman, R. T., & Fidock, D. A. (2009). Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nature reviews. Microbiology, 7(12), 864–874. https://doi.org/10.1038/nrmicro2239
- Vestergaard LS, Ringwald P. Responding to the Challenge of Antimalarial Drug Resistance by Routine Monitoring to Update National Malaria Treatment Policies. In: Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.
- Ashley, E. A., Dhorda, M., Fairhurst, R. M., Amaratunga, C., Lim, P., Suon, S., … & White, N. J. (2014). Spread of artemisinin resistance in Plasmodium falciparum malaria.New England Journal of Medicine, 371(5), 411-423.
About the Authors:
Bernard Owusu Agyare, MPH is a doctoral student in Global Infectious Disease, at Georgetown University and is also affiliated with the Center for Global Health Science and Security (CGHSS) at Georgetown University. Bernard is a global health practitioner and scholar with over 15 years of experience in Public Health, focusing particularly on infectious diseases outbreak preparedness and response in conflict settings. With prior training in Medical Laboratory, Bernard has extensive experience in malaria diagnosis in Sub-Sahara Africa.
Arielle Dolegui, MPH is a doctoral student in Global Infectious Disease, at Georgetown University and is also affiliated with the Center for Global Health Science and Security (CGHSS) at Georgetown University. Arielle is a global health practitioner with over 13 years of experience managing complex health systems strengthening programs and implementing strategic approaches with results-oriented program design, implementation, and evaluation expertise supporting neglected tropical diseases (NTD) and other public health programs
Jesus Aquilar is a Master of Public Health (MPH) student in the Global Infectious Disease program (GLID) at Georgetown University. Jesus research interest include exploring multidisciplinary approaches to malaria eradication in Low and Middle Income Countries (LMICs).
Leave a Reply
You must be logged in to post a comment.