By Matt Boyce, Gretchen Mohr, and Eva Rest
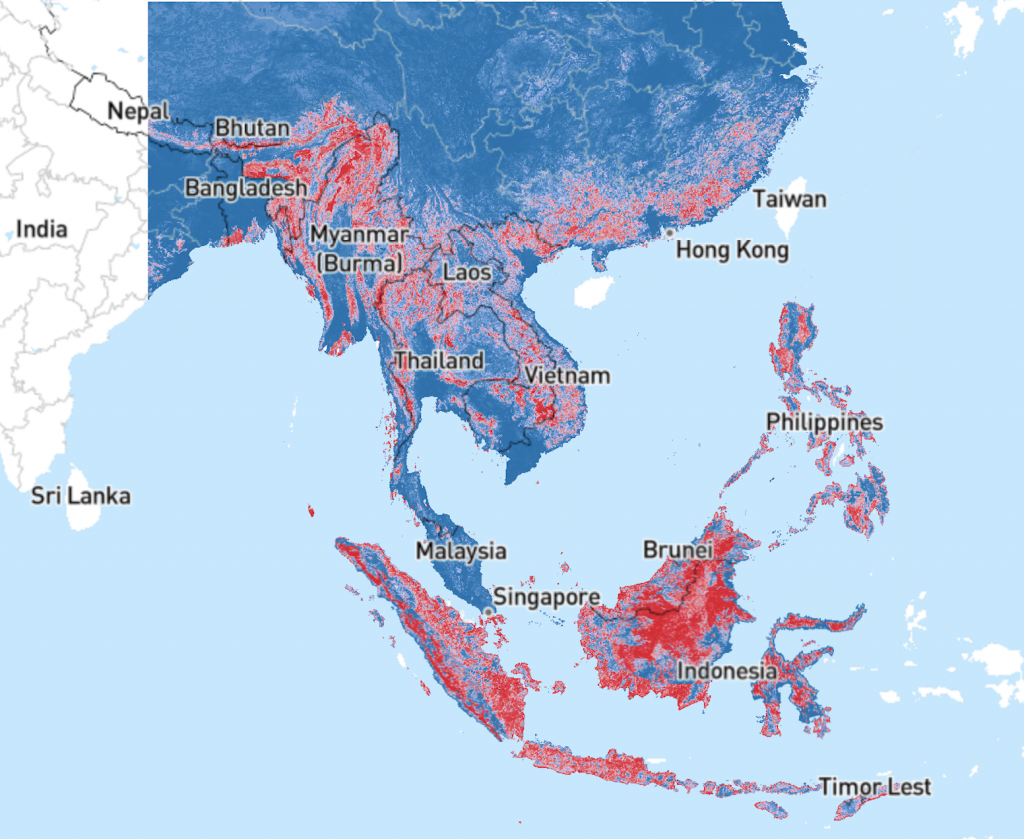
Plasmodium knowlesi, one species of the multiple protozoan parasites that cause malaria, has joined the lineup of human malaria parasites. P. knowlesi was originally known to cause malaria in long-tailed and pig-tailed macaques typically found in Southeast Asia (Figure 1). Only within the last two decades have scientists recognized that P. knowlesi is one of a few Plasmodium species that can also cause malaria in humans. Since that time, human malaria caused by P. knowlesi infections have been reported in almost every Southeast Asian country and in travelers returning from the region.
To understand this emerging public health threat, we will start at the beginning: where does this parasite originate? We also briefly discuss the distribution, the need for improved diagnostics, and how the emergence of P. knowlesi could interact with other factors to threaten efforts to eliminate the deadly disease.
Where did this parasite come from?
While it was only recently that we recognized the extent to which P. knowlesi causes human malaria, we have known about the parasite for much longer — since it was first isolated in 1931 from a long-tailed macaque. For decades, it was thought that P. knowlesi was one of the hundreds of known species of Plasmodium that infect other vertebrates, but not humans.
Deforestation for agriculture and logging has caused humans to interact more closely with macaques and their natural environment. As human activities and settlements expand farther into previously untouched areas, the high genetic diversity of P. knowlesi has allowed it to infect multiple hosts — humans and macaques. While currently thought to be most common in Malaysia, human infections with P. knowlesi have been reported across Southeast Asia. It is now recognized as the fifth known species to cause human malaria. As a result, it can be said that P. knowlesi is emerging — both literally and figuratively.
Infection with P. knowlesi can occur with smaller numbers of parasites than other human malaria species, but rapid reproduction can lead to high parasitemia and more severe clinical illness increasing with parasite density in the blood. P. knowlesi has the shortest erythrocytic cycle (i.e., reproductive cycle) of any Plasmodium species. This means within 24 hours of infection, the parasite can asexually reproduce. Like other Plasmodium, P. knowlesi can cause a variety of symptoms including fever, chills, nausea, anemia, jaundice, and death in severe cases. Fortunately, if it is detected early enough, infections can be treated with artemisinin-combination therapy for mild cases or intravenous artesunate for more serious cases.
What is happening now?
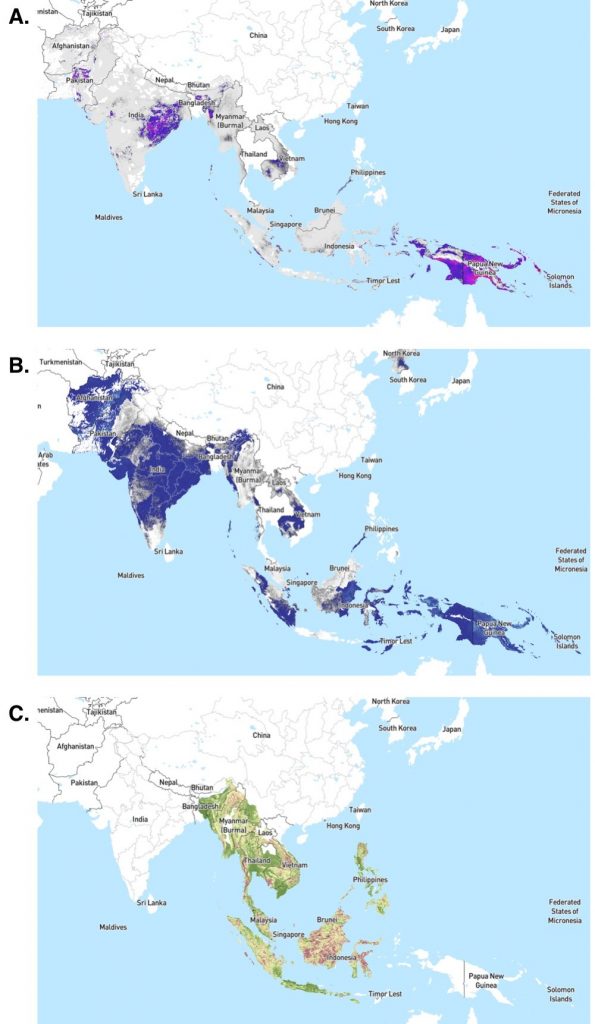
The geographic distribution of P. knowlesi in regions also endemic for P. vivax and P. falciparum makes malaria a triple threat. P. knowlesi frequently occurs as a co-infection with these more common human malaria parasites, further complicating efforts to understand its true distribution and impact (Figure 3).
The Anopheles mosquito vectors transmitting P. knowlesi are somewhat geographically limited, relative to other Plasmodium species, and commonly bite other primates. Thus most transmission to date has been zoonotic, involving an infected primate and a susceptible human linked by an infected Anopheles mosquito. Transmission between humans is relatively rare, but might increase if humans largely outnumber macaques in forests or habitats infested with mosquitoes. Already, some household clusters of the disease have been observed, indicating possible transmission close to human settlements.
The major Anopheles vectors of P. knowlesi tend to not rest or feed within houses. Although this makes use of insecticide-treated bed nets and indoor residual spraying less effective, it also limits biting and potential transmission to more remote areas. However, should P. knowlesi adapt to additional Anopheles species with broader geographic ranges, P. knowlesi malaria incidence could rapidly increase. As P. knowlesi becomes more established in humans, their Anopheles vectors may face selection pressure to live or feed closer to human habitation. The unique characteristics of P. knowlesi and its predominant Anopheles vectors may require additional resources to develop and implement targeted control strategies, distinct from strategies for other Plasmodium species.
What should we do?
Our increased awareness of the threats posed by P. knowlesi malaria may be explained by a variety of factors including improved diagnostic capacities, limited immunity in humans due to historically low transmission of P. knowlesi malaria, and changes in land use patterns increasing the opportunity for disease spillover. Regardless of the reason, acknowledging P. knowlesi malaria and addressing the emerging health challenge will undoubtedly require decisive policy action — especially if better control of malaria or the goals of malaria elimination are to be realized.
One component central to this will be more affordable and accessible diagnostic tools for P. knowlesi infections in humans. Before it was recognized as a human health threat, misdiagnosis — typically with P. malariae — was common and associated with more severe clinical consequences. This is because P. malariae and P. knowlesi are not reliably distinguished by microscopy — the gold standard for malaria diagnosis. In turn, more resource-intensive methods such as polymerase chain reaction (PCR) became the definitive diagnostic method for P. knowlesi malaria. While rapid malaria tests do address the shortcomings of other diagnostic approaches and improve the diagnosis of other types of malaria in resource-constrained settings, they have displayed suboptimal performance in detecting P. knowlesi malaria.
Accordingly, improving the diagnosis of P. knowlesi represents an important research and policy priority. A 2017 meeting of the World Health Organization’s Malaria Policy Advisory Committee noted several key diagnostic priorities for P. knowlesi including the development of new rapid diagnostic tests, selection of serological markers to assess human transmission intensities, and development of quantitative PCR as a means of determining what proportion of the population is infected with P. knowlesi.
Successfully pursuing these objectives could not only improve our understanding of the extent and distribution of the threats posed by P. knowlesi — improving our ability to make informed, evidence-based decisions — but could also yield very real benefits for human health in areas with multiple Plasmodium species circulating in the environment.
What does the future look like?
As countries progress toward malaria elimination, P. knowlesi poses an alarming risk of maintaining malaria within animal reservoirs. This would mean even detailed surveillance and successful treatment with antimalarial drugs might not be enough to eliminate malaria — as exclusive infection of a single host has traditionally been considered a necessity for eradication or elimination. Human settlements, labor, and resource extraction continue to spread farther into forested areas and meet the ‘fringe’ areas where P. knowlesi transmission already occurs. Accordingly, implementing and improving surveillance, diagnostic tools, and vector control strategies will be vital for addressing this emerging threat and modifying elimination efforts to limit resurgence.
Leave a Reply
You must be logged in to post a comment.